Abstract
Background: Retrospective studies suggest that change in ctDNA levels after 1-2 cycles of induction therapy in patients (pts) with previously untreated DLBCL is associated with event-free survival and overall survival (OS), using a log-fold change (LFC) cutoff in ctDNA levels of 2.0 (Kurtz DM, et al. J Clin Oncol 2018). The prognostic value of ctDNA has not been prospectively validated; this is a prespecified exploratory analysis of the prognostic value of ctDNA in the POLARIX study (NCT03274492).
Methods: Pts in POLARIX had previously untreated DLBCL and were randomized to receive Pola-R-CHP or R-CHOP (Tilly H, et al. N Engl J Med 2022). Plasma ctDNA levels were measured at baseline and Cycle 2 Day 1 (C2D1) using the AVENIO NHL CAPP-Seq assay and are reported as mean mutant molecules per mL (MMPM; Herrera AF, et al. Blood Adv 2022). Plasma-depleted whole blood at baseline was used as a source of germline DNA to filter non-tumor-specific variants. Change in ctDNA levels was characterized as the log10 ratio between baseline and C2D1 MMPM (LFC); a LFC cutoff of 2.0 was used in this analysis. ctDNA clearance was determined with a detection cutoff of p=0.005 (Scherer F, et al. Sci Transl Med 2016). Univariate and multivariate Cox regression were used to identify relationships between ctDNA and progression-free survival (PFS) and OS. Hazard ratios (HR) are reported with 95% confidence intervals.
Results: Results for paired ctDNA samples at baseline and C2D1 were available for 319/440 (72.5%; Pola-R-CHP) and 299/439 (68.1%; R-CHOP) pts. A median 135 (range: 1-644) variants were detected per pt. Baseline ctDNA levels were not statistically different between the two arms (median MMPM: Pola-R-CHP, 356; R-CHOP, 237; p=0.2); high baseline MMPM was associated with high baseline IPI score (p<0.001), ABC DLBCL (p<0.001), ECOG performance status >1 (p<0.001), and presence of bulky disease (>7.5cm; p<0.001). In both arms, pts with baseline ctDNA levels above the median MMPM had shorter PFS (Pola-R-CHP: HR, 1.96 [1.21-3.18]; R-CHOP: HR, 1.43 [0.92-2.20]) and OS (Pola-R-CHP: HR, 2.17 [1.07-4.37]; R-CHOP: HR, 2.10 [1.05-4.21]) than pts with baseline ctDNA levels below the median MMPM. Change in ctDNA levels from baseline to C2 as a continuous fold-change value was associated with PFS (HR, 0.75 [0.65-0.87]) and OS (HR, 0.78 [0.63-0.96]) in both arms. Pts with greater reductions in ctDNA levels after one treatment cycle had longer PFS and OS than pts with lower ctDNA reductions after one cycle.
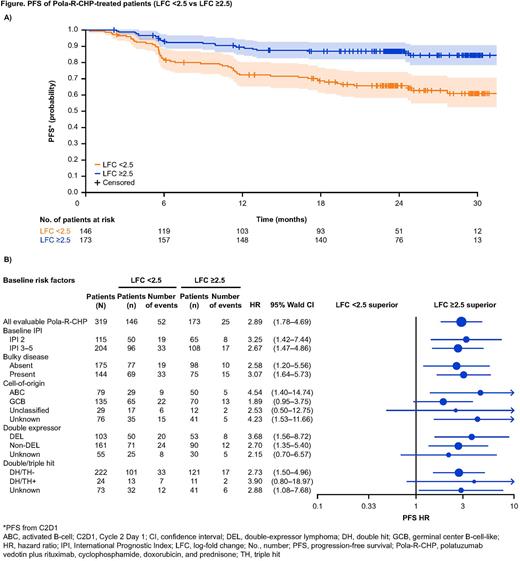
LFC ≥2.0 or undetectable ctDNA at C2D1 were achieved by 66.8% (213/319) of Pola-R-CHP-treated pts and 65.6% (196/299) of R-CHOP-treated pts. Stratifying pts at this LFC threshold was strongly prognostic of survival outcomes by arm (LFC <2.0 vs LFC ≥2.0, PFS HR: Pola-R-CHP 1.86 [1.18-2.92], R-CHOP 2.21 [1.43-3.41], OS HR: Pola-R-CHP 1.48 [0.77-2.81], R-CHOP 2.67 [1.34-5.30]). To define an optimal risk cutoff for pts receiving Pola-R-CHP, pts were grouped into training and validation cohorts. A LFC threshold of 2.5 (LFC ≥2.5 Pola-R-CHP: 54.2% [173/319]; R-CHOP: 54.8% [164/299]) was better able to identify pts at risk of poorer outcomes (LFC <2.5 vs LFC ≥2.5, PFS HR 2.89 [1.78-4.69], 24-month estimates 65.7% [58.3-74.0] vs 87.0% [82.0-92.2]; OS HR 1.87 [0.98-3.58], 24-month estimates 86.2% [80.8-92.0] vs 91.8% [87.7-96.0]; Figure A). In the Pola-R-CHP arm, pts with ctDNA clearance at C2D1 had superior outcomes vs pts with detectable ctDNA at C2D1 (not cleared vs cleared, PFS HR 2.98 [1.53-5.80]; OS HR 2.74 [1.07-7.02]). LFC 2.5 and ctDNA clearance were evaluated in separate multivariate analyses; it was found that both LFC 2.5 and ctDNA clearance are prognostic for PFS and OS in Pola-R-CHP treated pts, independent of key baseline risk factors (Figure B). LFC 2.5 and ctDNA clearance were also prognostic in R-CHOP-treated pts. Lastly, the top five commonly mutated genes identified in baseline ctDNA were HIST1H variants, TP53, PIM1, MYD88, and BCL2; analysis of clonal change on- and post-treatment is ongoing.
Conclusions: Based on this prespecified exploratory analysis from the POLARIX study, ctDNA analysis has prognostic value for pts with previously untreated DLBCL. Pts who did not achieve ≥2.5 LFC and/or did not have ctDNA clearance following one cycle of Pola-R-CHP had inferior outcomes than those who did. Early changes in ctDNA levels may be of use in risk-adapted trial designs to identify pts in need of alternative treatment.
Disclosures
Herrera:Takeda: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Pfizer: Consultancy; Seattle Genetics: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Genmab: Consultancy; KiTE Pharma: Research Funding; Tubulis: Consultancy; AstraZeneca: Consultancy, Research Funding; Adicet Bio: Consultancy; Caribou: Consultancy; Merck: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Regeneron: Consultancy; Gilead: Research Funding; Karyopharm: Consultancy. McCord:Genentech: Current Employment, Current equity holder in publicly-traded company. Kimes:Genentech/Roche: Current Employment; Dana-Farber Cancer Institute: Ended employment in the past 24 months. Lenz:Miltenyi: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Constellation: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Genmab: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Bayer: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; University Hospital Münster: Current Employment; Takeda: Honoraria, Speakers Bureau. Trneny:Novartis: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Zentiva: Consultancy, Honoraria. Flowers:Guardant: Research Funding; National Cancer Institute: Research Funding; Burroughs Wellcome Fund: Research Funding; Genentech/Roche: Consultancy, Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Allogene: Research Funding; Amgen: Research Funding; SeaGen: Consultancy; Karyopharm: Consultancy; Genmab: Consultancy; Cellectis: Research Funding; Morphosys: Research Funding; Celgene: Consultancy, Research Funding; Denovo Biopharma: Consultancy; Sanofi: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Janssen Pharmaceutical: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Kite: Research Funding; TG Therapeutics: Research Funding; Takeda: Research Funding; V Foundation, Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research: Research Funding; EMD: Research Funding; NPower: Current holder of stock options in a privately-held company; Adaptimmune: Research Funding; Xencor: Research Funding; Ziopharm: Research Funding; 4D: Research Funding; Acerta: Research Funding; Iovance: Research Funding; Spectrum: Consultancy; Pharmacyclics/Janssen: Consultancy; Gilead: Consultancy, Research Funding; BeiGene: Consultancy; Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding. Sehn:Teva, Roche/Genentech: Consultancy, Honoraria, Research Funding; Chugai: Consultancy, Honoraria; AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Honoraria; AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Debiopharm, Genmab, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Novartis, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Consultancy. Salles:AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy; Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria. Sharman:Beigene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Lilly: Consultancy, Honoraria, Research Funding; Araris Biotech AG: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Research Funding; Merck: Consultancy; Genentech: Consultancy; Pharmacyclics LLC, an AbbVie Company: Honoraria; BMS: Consultancy, Research Funding. Tilly:Incyte: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding. Herbaux:Abbvie: Honoraria, Research Funding; MSD: Research Funding; Roche: Honoraria; Janssen: Honoraria; Gilead: Honoraria; Kite: Honoraria; Takeda: Honoraria, Research Funding. Matasar:Seattle Genetics: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Current equity holder in private company; Rocket Medical: Consultancy, Research Funding; Takeda: Consultancy, Honoraria; Teva: Consultancy; GlaxoSmithKline: Honoraria, Research Funding; ADC Therapeutics: Consultancy, Honoraria; Daiichi Sankyo: Consultancy; F. Hoffmann-La Roche Ltd.: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy; Juno Therapeutics: Consultancy; ImmunoVaccine Technologies: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; IGM Biosciences: Research Funding; TG Therapeutics: Consultancy; Karyopharm: Consultancy; IMV Therapeutics: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria. Haioun:BMS: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Miltenyi Biotec: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Tracy:F. Hoffmann-La Roche: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Hirata:Roche: Current holder of stock options in a privately-held company; Genentech, Inc.: Current Employment. Lee:Roche: Current equity holder in publicly-traded company; Genentech: Current Employment. Jiang:Roche: Current equity holder in publicly-traded company; Roche/Genentech: Current Employment. Morschhauser:Miltenyi: Membership on an entity's Board of Directors or advisory committees; Janssen: Speakers Bureau; Genentech: Consultancy; Genmab: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Allogene therapeutics: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Polatuzumab vedotin (Polivy) is a CD79b-directed antibody-drug conjugate indicated in combination with bendamustine and a rituximab product for the treatment of adult pts with relapsed or refractory DLBCL, not otherwise specified, after at least two prior therapies.Rituximab (Rituxan) is a CD20-directed cytolytic antibody indicated for the treatment of adult pts with: relapsed or refractory, low grade or follicular, CD20-positive, B-cell NHL as a single agent; previously untreated follicular, CD20-positive, B-cell NHL in combination with first-line chemotherapy (chemo) and, in pts achieving a CR or PR to a rituximab product in combination with chemo, as single-agent maintenance therapy; non-progressing (including stable disease), low-grade, CD20-positive, B-cell NHL as a single agent after first-line CVP chemo; previously untreated diffuse large B-cell, CD20-positive, NHL in combination with CHOP or other anthracycline-based chemo regimens; previously untreated and previously treated CD20-positive CLL in combination with fludarabine and cyclophosphamide.
Author notes
Asterisk with author names denotes non-ASH members.